
- We will send in 10–14 business days.
- Author: Fatima Haj Ahmad
- Publisher: Cuvillier
- ISBN-10: 3736970463
- ISBN-13: 9783736970465
- Format: 14.8 x 21 x 1 cm, softcover
- Language: English
- SAVE -10% with code: EXTRA
Phosphoproteomics Analysis of the Systemin Signaling Pathway (e-book) (used book) | bookbook.eu
Reviews
Description
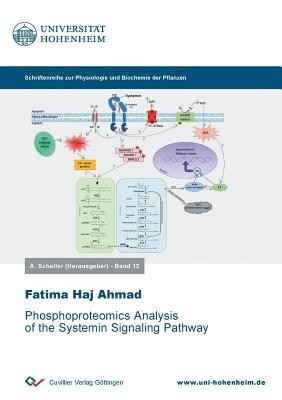
Systemin is a small peptide with important functions in plant wound response signaling. To elucidate systemin perception and signal transduction mechanisms, a phosphoproteomic profiling study was performed to reconstruct a systemin-specific kinase/phosphatase signaling network. Time course analyses revealed early events at the plasma membrane, such as dephosphorylation of H+-ATPase, and the phosphorylation of NADPH-oxidase and Ca2+-ATPase in response to systemin. Later responses included transient phosphorylation of small GTPases and vesicle trafficking proteins, as well as transcription factors. Based on a correlation analysis of systemin-induced phosphorylation profiles, substrate candidates for 44 systemin-responsive kinases and 9 phosphatases were predicted, some of which are involved in a regulatory circuit for the regulation of the plasma membrane H+-ATPase. In this regulatory model, H+-ATPase LHA1 is rapidly de-phosphorylated at its C-terminal regulatory residue T955 by phosphatase PLL5, resulting in the alkalization of the growth medium within two minutes of systemin treatment. LHA1 is re-activated by MAP-Kinase MPK2 later in the systemin response. A valuable resource of proteomic events involved in the systemin signaling cascade is provided with a focus on the prediction of substrates to early systemin-responsive kinases and phosphatases.
EXTRA 10 % discount with code: EXTRA
The promotion ends in 20d.17:38:53
The discount code is valid when purchasing from 10 €. Discounts do not stack.
- Author: Fatima Haj Ahmad
- Publisher: Cuvillier
- ISBN-10: 3736970463
- ISBN-13: 9783736970465
- Format: 14.8 x 21 x 1 cm, softcover
- Language: English English
Systemin is a small peptide with important functions in plant wound response signaling. To elucidate systemin perception and signal transduction mechanisms, a phosphoproteomic profiling study was performed to reconstruct a systemin-specific kinase/phosphatase signaling network. Time course analyses revealed early events at the plasma membrane, such as dephosphorylation of H+-ATPase, and the phosphorylation of NADPH-oxidase and Ca2+-ATPase in response to systemin. Later responses included transient phosphorylation of small GTPases and vesicle trafficking proteins, as well as transcription factors. Based on a correlation analysis of systemin-induced phosphorylation profiles, substrate candidates for 44 systemin-responsive kinases and 9 phosphatases were predicted, some of which are involved in a regulatory circuit for the regulation of the plasma membrane H+-ATPase. In this regulatory model, H+-ATPase LHA1 is rapidly de-phosphorylated at its C-terminal regulatory residue T955 by phosphatase PLL5, resulting in the alkalization of the growth medium within two minutes of systemin treatment. LHA1 is re-activated by MAP-Kinase MPK2 later in the systemin response. A valuable resource of proteomic events involved in the systemin signaling cascade is provided with a focus on the prediction of substrates to early systemin-responsive kinases and phosphatases.

Reviews